教育经历
· 1997.8 - 2004.1
University of Virginia - Biophysics - Ph.D.
· 1993.8 - 1996.7
Tsinghua Universiy - Biophysics - M.S.
· 1987.8 - 1991.7
Peking University - Physics - B.S.
工作经历
· 2014.10 - 2019.12
School of Life Sciences → Tianjin University → Professor
· 2005.1 - 2014.9
Immune Disease Institute → Harvard Medical School → Research Fellow
· 2004.1 - 2005.1
Department of Pharmacology → University of Virginia → Research Associate
· 1997.8 - 2003.12
Biophysics Program → University of Virginia → Research Assistant
· 1993.8 - 1997.7
Department of Biology → Tsinghua University → Research Assistant
· 1991.8 - 1993.7
Department of Basic Sciences → Tianjin Sino-German Vocational Institute of Technology → Teaching Assistant
研究方向
· Conformational coupling through a single pass transmembrane domain in receptor tyrosine kinases
· Structural and functional basis for developmental signaling
社会兼职
-
· 2017.8-至今
Chinese Cell Biology Society, Chapter of Tianjin, Board Counsellor
-
· 2017.6-至今
Chinese Biophysical Society, Chapter of Cryo-EM, Board Counsellor
个人简介
Dr. Mi is interested in the structural and mechanistic basis of developmental signaling, which is important in stem cell biology, cancer metastasis, and regenerative medicine. Especially, he focuses on understanding how the activities of key developmental signaling molecules are regulated by extracellular micro-enviroments, and how these molecules are recognized by cell surface receptors to engage with intracellular signaling cascades through lipid membrane. In addition, he wants to translate his structural understanding into therapeutic development against cancer and degenerative diseases.
Conformational coupling through a single pass transmembrane domain in receptor tyrosine kinases
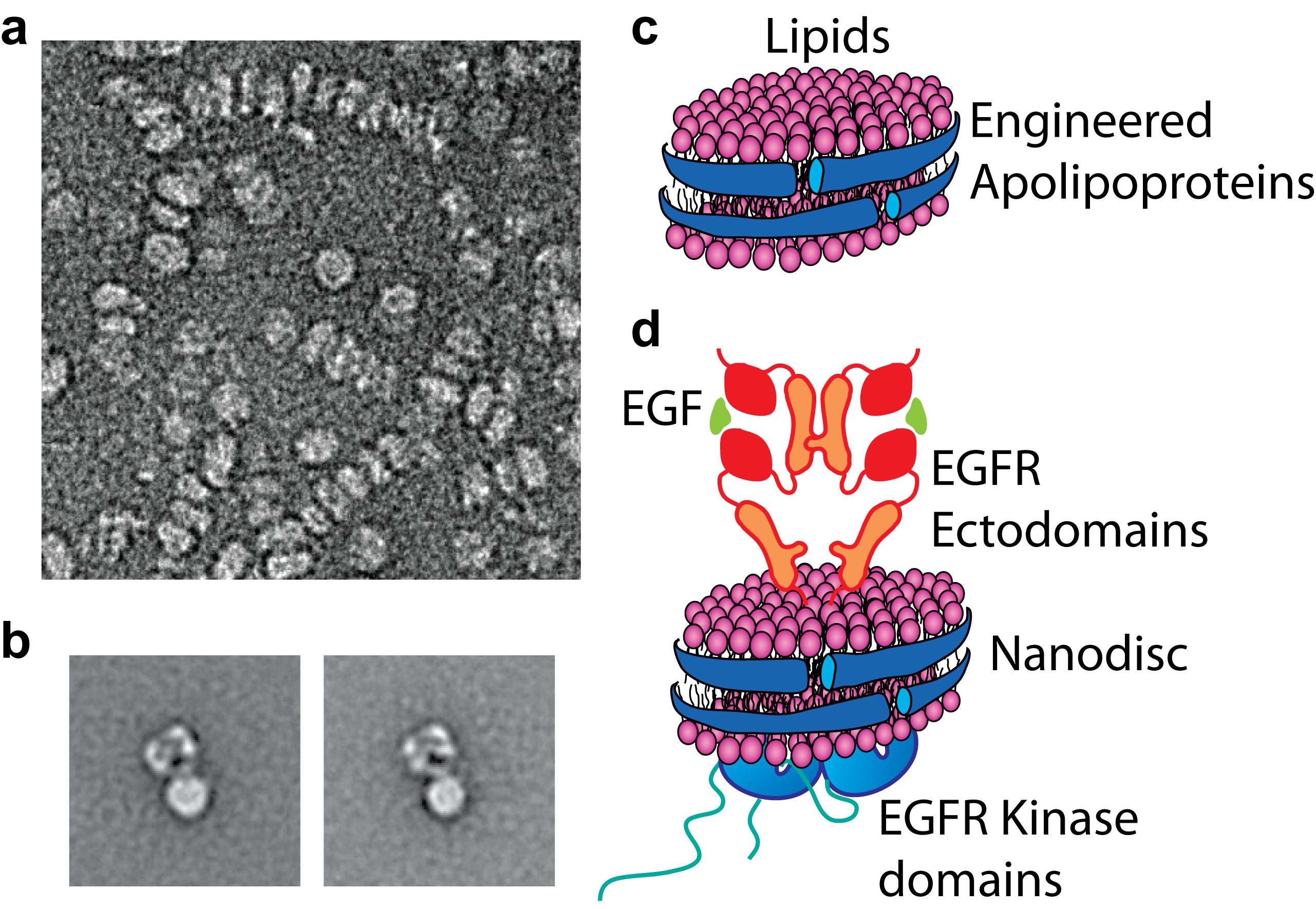
We focused on the understanding how external signals are recognized by cell surface receptors and transmitted through lipid membrane to engage intracellular signaling. Specifically, we want to understand how ligand recognition is coupled to intracellular signaling in an intact receptor, especially in those receptors with a single-pass transmembrane domain. As the first step to appreciate this question, we visualized, in a nearly full-length EGF receptor, the communication between the ectodomain and the cytoplasmic domain by electron microscopy. We observed that one conformation of liganded EGF receptor ectodomain can be coupled to multiple cytoplasmic (kinase) domain arrangements. Moreover, the activated, asymmetric kinase domain dimer can be coupled to two ectodomain association states depending on the presence of ligand (Mi et. al., NSMB 2011). This unexpected loose coupling across the membrane facilitates regulation of the EGF receptor function in the juxtamembrane and cytoplasmic environments.
Figure 1: Functional reconstitution of membrane proteins into lipid nanodiscs. (a) raw EM image of reconstituted empty nanodiscs. (b) class averages of the EGF receptors reconstituted in nanodiscs. (c,d) Schematic diagrams of the reconstituted empty (c) and EGFR-loaded (d) nanodiscs. In my studies, the EGF receptors reconstituted in nanodiscs are structurally and functionally stable.
学术成果
奖励与荣誉
2. Tsinghua University, ”Yiqi Mei” President Fellowship, 1995
3. Tsinghua University, The Second Prize in Challenger Cup Scientific Competition, 1996
5. Harvard Medical School, Poster Award in 2007 Immune Disease Institute summer retreat, 2007
6. Harvard Medical School, Poster Award in 2010 Immune Disease Institute summer retreat, 2010




